Giancarlo Barolat, MD
Danielle Axe was a healthy 20 year old female when in 2000 she sustained a fall and struck her back in the interscapular area. She started to complain of continuous burning pain that is  characteristic of RSD. The area between the shoulder blades became painful, and a smaller area centered over the spinous processes of T5, T6, and T7 developed severe sensitivity to light touch in the affected region (allodynia). She could not wear a bra, since the mechanical stimulation caused excruciating pain.
characteristic of RSD. The area between the shoulder blades became painful, and a smaller area centered over the spinous processes of T5, T6, and T7 developed severe sensitivity to light touch in the affected region (allodynia). She could not wear a bra, since the mechanical stimulation caused excruciating pain.
The patient underwent extensive investigations, but no structural lesion could be found. In order to minimize the pain, Danielle began relying on powerful narcotic medications. The medications, however, barely touched the intense pain. The symptoms had significantly increased over time to the point where she had to use a walker to even get around.
I saw Danielle in my office in 2002 when she was 7 months pregnant. This was a very challenging case. Her pain was purely axial, occupying a 10 cm square area between the shoulder blades. Spinal cord stimulation was not an option, since that specific area can not be covered by the stimulation. Intrathecal narcotic administration was not an optimal choice either, because of her young age and her desire to have more children.
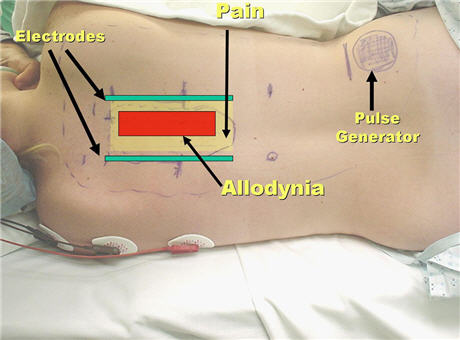
I decided to perform a temporary test trial with externalized electrodes implanted subcutaneous tissues of the posterior thoracic area. At that time I had only performed a handful of those types of implants, and never in a situation like this, a patient with interscapular pain and allodynia. 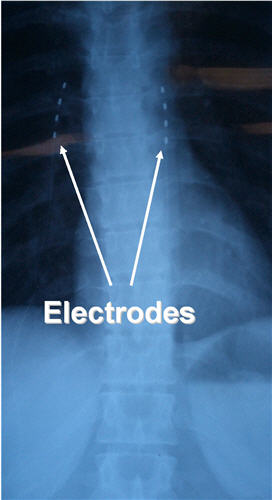 I was not exactly sure about the best construct for the implantation of the electrodes. I decided to insert one electrode in the midline, exactly in the area of allodynia, and two other electrodes at the borders of the painful area. The procedure was accomplished uneventfully.
I was not exactly sure about the best construct for the implantation of the electrodes. I decided to insert one electrode in the midline, exactly in the area of allodynia, and two other electrodes at the borders of the painful area. The procedure was accomplished uneventfully.
When the stimulation was turned on, it was clear that activation of the midline electrode was actually increasing the pain. A small miracle occurred when the two lateral electrodes were activated: The severe constant pain was markedly reduced and the extreme sensitivity to light touch almost completely disappeared. The results were confirmed by several days of trial stimulation with the wires from the electrodes externalized through the skin to a battery (an externalized trial).
One month after successful delivery of a healthy baby girl, Danielle underwent surgical implantation of two percutaneous electrodes in the interscapular area with an attached implanted pulse generator. The pulse generator is controlled by an external remote device; through this device the stimulator can be turned on or off and some of the parameters (voltage, rate, etc) can be modified. This gives the patient full control over the various stimulation settings and allows the patient to “tailor” the stimulation to their pain needs. Some patients need to activate the stimulator only when the pain becomes unbearable. Other patients find it more useful to have it running all the time, in order to prevent the severe pain from occurring.
Ever since implantation of the device, Danielle has been almost completely pain free. The exquisite sensitivity to light touch disappeared. During the past couple of years she has been completely off all medication. Her life has changed so drastically, that she decided to go ahead and become pregnant again. Danielle uses the stimulator every day continuously, in order to maintain the pain-free status. This has enabled her to conduct a normal pregnancy.
A recent quote from her is “I have been given a second chance at life”.
Other successful applications of subcutaneous peripheral neurostimulation include headaches, atypical facial pain, intractable low back and neck pain following failed spine surgery as well as other types of fairly well localized pain syndromes that have not responded to other forms of treatment. The role of these modalities in the management of severe chronic pain conditions is in progress, and they should be considered only when more conventional and non-invasive treatments have failed.
EDITOR’S NOTE:
Giancarlo Barolat, MD, has wriiten an excellent case history and is to be congratulated on his achievement with this most difficult patient.
Dr. Barolat finished medical school in 1974 at the University of Torino, Italy. He is certified by both the American and the Italian Board of Neurosurgery.
Dr. Barolat was President of the International Neuromodulation Society and is on the Board of the American Neuromodulation Society and on the Editorial Board of the Journal, Neuromodulation. He is currently Director at large of the International Neuromodulation Society.
Neuromodulation Society and on the Editorial Board of the Journal, Neuromodulation. He is currently Director at large of the International Neuromodulation Society.
Dr. Barolat practiced at Thomas Jefferson University Hospital from 1985 to 2004 as Professor of Neurosurgery and Director of the Division of Functional Neurosurgery. Dr. Barolat has extensive experience with surgical spine procedures. As such, he has performed hundreds of complex spine operations. He has also been involved in the surgical management of intractable seizures through implantation of vagus nerve stimulator devices.
Dr. Barolat is also one of the world leaders in the area of neuro-implantable technologies for the management of pain and motor disorders. He is one of the pioneers of spinal cord stimulation for spasticity and pain management. His practice is one of the largest in the country, with national and international referrals. He has authored of over 60 medical articles and book chapters. He has lectured extensively nationally and internationally.
Dr Barolat is currently practicing neurosurgery and neuromodulation in Denver, Colorado, and is affiliated with Skyridge Medical Center. He is the CEO and Director of the Barolat Institute.
We thank Phil Spiegel, MD, and Enrico Camporesi, MD, for their review and constructive comments on Dr. Barolat's case report.
Anthony Kirkpatrick, MD, PhD

