How
to Determine the Effectiveness
of Treatments for RSD / CRPS
Dr. Anthony F. Kirkpatrick,
Chairman, Scientific Advisory Committee
The first thing that should be understood about
treating any medical condition is there are three
things that can lead to improvement in the condition:
- Natural History of the
Disease
- Placebo or Nonspecific
Effects of Treatment
- Specific Effects of Treatment
The natural history of a disease
can be highly variable. A headache comes and goes,
as does a backache. Similarly, the symptoms of RSD
can come and go, especially in children. Many adults
who have RSD report that the pain tends to be there
constantly, but the level of pain and symptoms goes
up and down. Thus the natural course of the disease
can in itself lead to temporary improvement in the
medical condition. When being treated for RSD, you
need to be able to separate what would happen anyway,
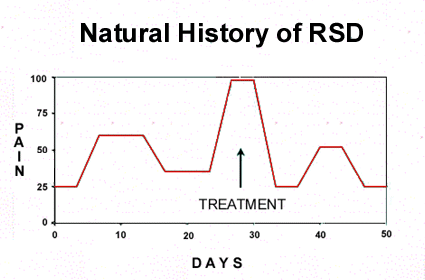
from what is being caused by the treatment. Figure
1 illustrates what is typical for RSD. The pain
goes along, then there is an exacerbation of symptoms,
it reaches a plateau or peak and then comes down
on its own without any treatment at all. The patient
seems to be on a roller coaster ride, but the pain
is always present at some level in most patients.
One of the things that seems predictable is when
there is an acute exacerbation of a disease (whether
it be a headache, low back pain or RSD), there is
a tendency for the doctor to treat the disease at
that point in time. But, this is when the symptoms
of a disease might decrease without treatment. Therefore,
when the symptoms of a disease fluctuate, the doctor
may take undue credit for the improvement following
his or her treatment.
Similarly, symptoms way worsen once treatment has
begun, even with an effective treatment. The doctor
may be blamed unfairly for a bad outcome following
his or her treatment. There are many factors that
go into the doctor-patient relationship, but too
often the patient classifies their treatment as
a failure and start doctor shopping. This often
results in patients dismissing traditional medicine
and adopting less conventional treatment.
Because the symptoms of RSD fluctuate, the Clinical
Practice Guidelines for RSD recommend that patients
be offered a "series" of 1-3 sympathetic
blocks.
With a series of sympathetic blocks, the patient
has a better chance of determining the true causes
of improvement or worsening of symptoms after treatment
in the context of a disease where there are spontaneous
fluctuations in symptoms.
FIGURE
1
 The placebo effect is due to
"treatments," which have no real physiological or
pharmacological property, that affect the disease
process. We call the placebo response to treatment
a nonspecific effect. For example, treatment with
inactive "sugar pills" can improve a patient's condition.
The patient needs to be able to separate the placebo
effect from the specific effects due to the pharmacological
or physiological action of the treatment on the
natural history of a disease. Medical insurance
companies and other third party payers are interested
in paying for treatments that really have a pharmacological
or physiological effect on the body. They do not
want pay for placebos. Everyone should be aware
and not wish to waste resources on these treatments.
The placebo effect is due to
"treatments," which have no real physiological or
pharmacological property, that affect the disease
process. We call the placebo response to treatment
a nonspecific effect. For example, treatment with
inactive "sugar pills" can improve a patient's condition.
The patient needs to be able to separate the placebo
effect from the specific effects due to the pharmacological
or physiological action of the treatment on the
natural history of a disease. Medical insurance
companies and other third party payers are interested
in paying for treatments that really have a pharmacological
or physiological effect on the body. They do not
want pay for placebos. Everyone should be aware
and not wish to waste resources on these treatments.
The response rate to placebos may vary depending
on the circumstances. Virtually anyone can respond
to a placebo if the conditions are right. Furthermore,
the effects of the placebo can be profound, resulting
in 100% relief of pain in some cases.
Many factors can influence the magnitude of the
placebo response:
- Anxiety
- Expectation
- Prestige of the doctor
- Cost and invasiveness of
the treatment
The more anxious you are, the
stronger your response to a placebo, especially
when the symptom is pain. 1 Patient expectation is also an important factor.
For example, one study looked at patient expectation
as a factor in treating a potentially life-threatening
symptom that causes difficulty in breathing in asthmatic
patients. 2 The life-threatening symptom is called bronchospasm.
The researchers found that when patients were told
they would receive either an inactive drug (placebo)
or an active drug for asthma, the placebo caused
little relief of bronchospasm. On the other hand,
if the researchers told the patients they were comparing
two "drugs" to determine which drug was more effective
against bronchospasm, the placebo (inactive drug)
had a much greater effect in relieving the symptom
of bronchospasm, than if the patients were told
one of the drugs was inactive. In other words, if
the patient had the expectation that the placebo
drug had some pharmacological action on asthma,
the placebo effect had a positive effect, even possibly
saving a life by decreasing in airway resistance
due to bronchospasm. The friendliness, sympathy,
empathy, and prestige of the doctor can also enhance
the beneficial effects of the placebo on the patient's
medical condition. 3,4
The expectation associated with a more invasive
or costly treatment can also enhance the placebo
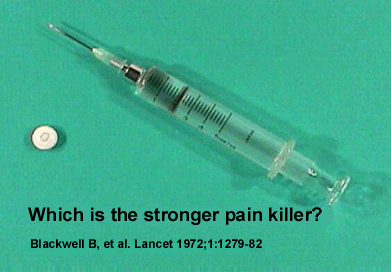
effect. 5 For example, take a look at the pill and syringe
in Figure 2. Both contain the same amount of pain
killer (an opioid narcotic). Which one of these
is the much stronger pain killer? The answer is
obvious. Doctors can legitimately take advantage
of this placebo effect when sedating patients for
various procedures to treat RSD,such as sympathetic
nerve blocks. The doctor may want the patient to
"see" them give the pain killer with a syringe in
the patient's IV. They may also want to "tell" the
patient they are injecting a "narcotic." The doctor
is not deceiving the patient because he is telling
the truth. The doctor knows the placebo effect can
be effective in relieving pain. The placebo effect
can decrease the amount of narcotic required for
the procedure and thereby minimize the narcotic's
side effects, such as poor breathing (hypoventilation)
during the procedure.
FIGURE
2
 Even the color of a placebo pill
can determine the nature of its effect on disease.
For example, white pills are more effective in treating
pain than yellow pills. On the other hand, yellow
pills are more effective than white pills in treating
depression. 6 Also, there can be a dose response to placebos where
two placebo pills are more effective than one placebo
pill. 5
Even the color of a placebo pill
can determine the nature of its effect on disease.
For example, white pills are more effective in treating
pain than yellow pills. On the other hand, yellow
pills are more effective than white pills in treating
depression. 6 Also, there can be a dose response to placebos where
two placebo pills are more effective than one placebo
pill. 5
It is a mistake to believe that placebos cannot
hurt you. In many studies patients demonstrate side
effects to placebos. On average, 20% of patients
have side effects from placebos that impact significantly
on their quality of life. These side effects include
drowsiness, headaches, nervousness, insomnia, nausea,
constipation. 7
Surgeries can have profound placebo effects. Back
in the late 1950s, two well-controlled studies (i.e.,
double-blind randomized trials) woke us up to this
reality. 8,9 Back then, it was believed that tying off (ligating)
the arteries in the chest wall would divert blood
from the muscle in the chest wall into the heart
and help relieve chest pain due to a heart condition
called angina. The surgery was called internal mammary
artery ligation. The results were astounding. Some
patients were getting profound relief of their chest
pain and improvement in their exercise tolerance
following this surgery. Some patients were getting
out of bed for the first time. Furthermore, some
patients had relief of their chest pain lasting
for over a year. However, some surgeons thought
that a control study should be performed to rule
out the placebo effect. Two studies were performed
where an incision was made in the chest wall but
the blood vessels were not tied off (a sham operation).
Guess what? They got the same results as when the
arteries were tied off. These results made it clear
that surgery can have a potent placebo effect.
Given that the placebo can have positive effects
on disease, why not intentionally deceive the patient
with inactive treatments?
- Deliberately deceiving
the patient will severely undercut the doctor-patient
relationship. If a doctor promotes a particular
treatment, and the patient learns the doctor
did not know whether the treatment was effective,
the patient may feel cheated by the doctor.
The patient may feel even greater resentment
toward the doctor if they suffer a serious complication
from a treatment with questionable effectiveness.
- As discussed, the placebo
effect can have significant adverse effects
that impact on the quality of life.
- The patient who is told
the drug is expected to work, but it doesn't
work in their particular case, may be harmed
by the fact that they are provoked into believing
their disease is much worse than it really is.
A negative prognosis provoked under these circumstances
may cause unnecessary anxiety in the patient.
- It is clear some patients
with RSD respond differently or not at all to
the same drugs. A good example is the use of
various antidepressants to treat RSD. If the
doctor tells the patient they should respond
to the drug (in order to enhance the placebo
effect), and they do not, you can have what's
called a negative carry over placebo effect,
so that any other treatment, even if effective,
may not work as well.
For all of these reasons, doctors
should not deliberately deceive patients with inactive
treatments in an effort to enhance the positive
effects of the placebo. There are four ways to help
determine the effectiveness of treatments for RSD:
- Education: The first
thing you can do is educate yourself about the
natural history of RSD and the importance of
the placebo effect.
- Monitor Response: The placebo effect over time tends to be less
effectiveness and shorter in duration with each
repeat of the treatment. For example, the placebo
effect tends to become less and less effective
with each repeat sympathetic block. 10,11 You really have to pay attention to the nature
of the response to each treatment. Unfortunately,
patients with chronic pain have difficulty remembering
things. Keeping a diary that documents the magnitude
and duration of the effect after each treatment
can help overcome this problem.
- Withdrawal Trials: Withdrawal trials are an important means of
ruling out placebo effects. You can wean yourself
off of the medication, or a particular treatment,
to see if it makes a difference. And, if it
seems to make a positive difference when you
are on the treatment, go back on it. If the
withdrawal trial makes a negative difference
(i.e., stopping the treatment stops a particular
side effect), you may need to stop the treatment.
The duration of the effectiveness of the treatment
needs to be considered. There are short-term
benefits for some treatments that no longer
exist once 6-9 months or more have passed. These
withdrawal trials may need to be repeated several
times to assure yourself of the treatment's
effectiveness. In some cases, the withdrawal
trial must be done slowly, especially if the
treatment is a narcotic (e.g., opioid, benzodiazapine)
or a potent muscle relaxant like baclofen. Otherwise,
you may suffer serious physical and/or psychological
withdrawal symptoms.
- Inquire about Research
on the Treatment: As much as possible, look
for clinical practice guidelines that are based
on well-controlled clinical trials.
A Withdrawal Trial:
The clonidine patch is an example
of where a withdrawal trial was used to determine
its effectiveness. 12 Clonidine seems to be a blocker of the sympathetic
nervous system. Many years ago researchers found
that if clonidine was applied to the skin of RSD
patients as a patch, the skin under the patch became
less sensitive to light touch. However, it was only
under the patch that the patient experienced the
decreased pain. The researchers concluded that the
clonidine patch would probably not be practical
for treating RSD because you could not have the
patient walking around with hundreds of patches
on their body. Several years ago, we thought of
taking a different approach to the clonidine patch.
We tried a higher dose and put the patch on the
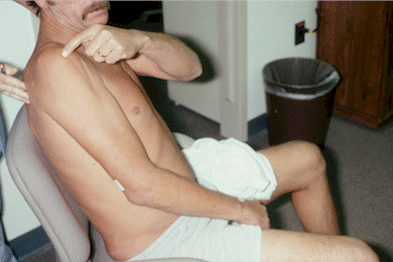
patient for a longer period of time. 13 Figure 3 shows a man who had painful, immobilized,
swollen and bluish discolored skin throughout his
entire upper extremity due to RSD. We applied two
clonidine patches to his extremity. Over time, the
pain, swelling and bluish discoloration almost disappeared.
Could this improvement be a placebo effect or simply
due to a change in the natural course of the disease?
What we did next was to take the patch off and see
what would happen. After two or three days, the
pain, swelling and discoloration returned and his
extremity became immobilized. After doing five or
six withdrawal trials, we (and the patient) became
reasonably convinced that the clonidine patch really
did work.
FIGURE
3
The "Gold Standard" in determining
the true causes of improvement in pain after treatment
remains the randomized controlled clinical trial.
Let's look at how the Scientific Advisory Committee
can help you determine if a treatment is effective.
Hot peppers can be made into a paste called capsaicin.
When applied to the skin, especially if the skin
is sensitive to light touch due to RSD, capaicin
causes a burning sensation. In addition, it causes
sneezing and, if you rub your eye after applying
it to your skin, it can cause a severe burning sensation
in the eye. If you keep on applying capsaicin to
the skin for a week or longer the burning sensation
will decrease because the skin becomes desensitized
to it. Capsaicin is believed to decrease the pain
of RSD by causing chemical changes in the nerve
endings in skin.
The Scientific Advisory Committee was confronted
with the question of whether capsaicin was effective
in treating RSD. In 1991, a rather impressive study
was published in a peer reviewed medical journal. 14 The title of the article was, "A multicenter double
blind vehicle controlled study: Capsaicin study
group." When you read a title like that you may
say, "hey, this study is going to tell me whether
or not this stuff really works." But if you take
a closer look at the study, you realize the study
isn't really "blinded." It is not a double blind
study because capsaicin burns when it is applied
to the skin, but the control paste (without capsaicin)
does not burn. Thus using "double blind" in the
title of the article is a misrepresentation because
the doctor and patient have a way of knowing if
the patient is getting capcaicin.
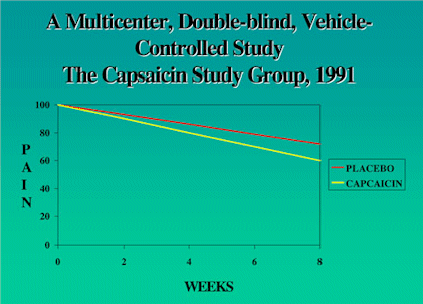
Figure 4 shows the results of the study. In both
the inactive drug control (vehicle) group and in
the capsaicin group there was a significant decrease
in pain over an eight weeks period. However, the
difference between the inactive drug group and the
capsaicin group is only the difference between a
pain score of 8 versus 7. That isn't a big difference
in pain score after having to apply this paste to
the skin three to four times per day for over eight
weeks. The results seem even less impressive if
one recalls that capsaicin actually causes a burning
sensation when applied initially and also causes
increase sneezing in the patient and others.
FIGURE
4
A group of neurologists at Mayo
Clinic decided to repeat the study, because they
wanted to know if capsaicin really works. This time
they did a truly double blinded study. They put
a substance called nicotinate into the paste for
the inactive drug control (vehicle) group. Nicotinate
causes a burning sensation when applied to the skin.
Now, the patient could not tell if they were receiving
the placebo control or capsaicin. As in the prior
study, both the placebo control group and capsaicin
group reported a decrease in pain, but there was
no difference in pain relief between the two treatment
groups. 15 Capsaicin is mentioned in the Clinical Practice
Guidelines because it is still used by some doctors
to treat RSD, but the Guidelines state there is
no data to support its efficacy in the treatment
of RSD.
CONCLUSIONS
The placebo effects of treating
RSD can be underestimated and patient improvement
may occur, at least temporarily, regardless of treatment.
Placebo effects and the natural history of RSD must
be distinguished from the specific effects when
medical and surgical treatments are evaluated. The "Gold Standard" in determining the true causes of
improvement in pain after treatment remains the
randomized controlled clinical trial. Doctors who
use inactive treatments in the hopes of producing
positive placebo effects run several risks.
|
|
|