The Centers for Medicare and Medicaid Services (CMS) introduced several new reimbursement codes for rechargeable neurostimulators that are  effective for services performed in the ambulatory surgery center (ASC) setting on or after January 1, 2006. In addition, CMS approved New Technology Add-On payments for rechargeable neurostimulators used in the hospital inpatient setting that became effective October 1, 2005.
effective for services performed in the ambulatory surgery center (ASC) setting on or after January 1, 2006. In addition, CMS approved New Technology Add-On payments for rechargeable neurostimulators used in the hospital inpatient setting that became effective October 1, 2005.
CMS staff on the Healthcare Common Procedure Coding System (HCPCS) Editorial Panel initially recommended against creation of the new rechargeable neurostimulator codes in April 2005. However, Richard North, MD, Director of Functional Spine Neurosurgery at Johns Hopkins University, Baltimore, testified to the HCPCS Editorial Panel on behalf of all of the neurostimulator manufacturers, which had coordinated to develop joint coding recommendations. CMS reconsidered and agreed to create the new codes.
Inadequacy of reimbursement for neurostimulator generators has historically limited Medicare patients' access to these devices in the ASC setting. Until now, Medicare reimbursement was highly inadequate for all neurostimulator generators in the ASC setting and did not differentiate between the existing nonrechargeable devices and the new rechargeable devices. As a result, ASC reimbursement for the rechargeable generators was at the same level as that for the less expensive nonrechargeable systems and did not cover the costs of either technology.
The CMS decision should expand patient access and stimulate conversion to rechargeable neurostimulators by aligning facilities' financial incentives to use the best technology with their desire to provide the highest quality of care for patients.
CMS is retiring the existing "E" codes for neurostimulators and replacing them with a new series of "L" codes that differentiate between different types of neurostimulators. By placing these codes in the "L" series, CMS is placing these devices in a coding category that is focused on implanted prosthetic devices. Complete lists of the new neurostimulator "L" codes as well as the retired "E" codes are shown in Tables 1 and 2. Codes for neurostimulators implanted in the hospital inpatient setting are shown in Table 3, and diagnosis codes related to the need for spinal cord stimulation are shown in Table 4.
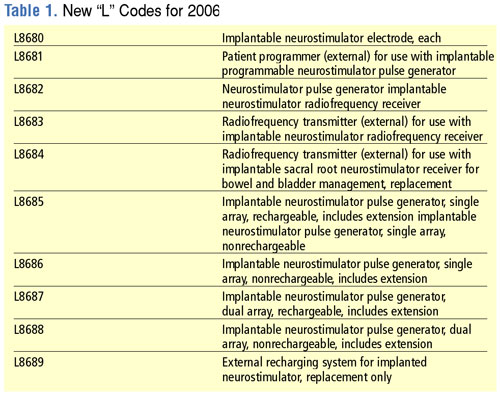
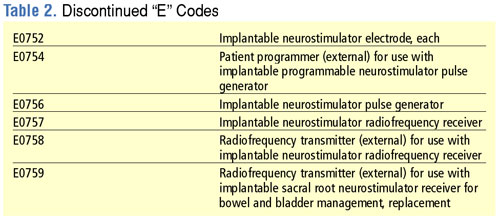
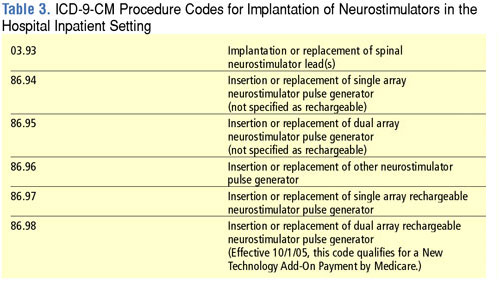
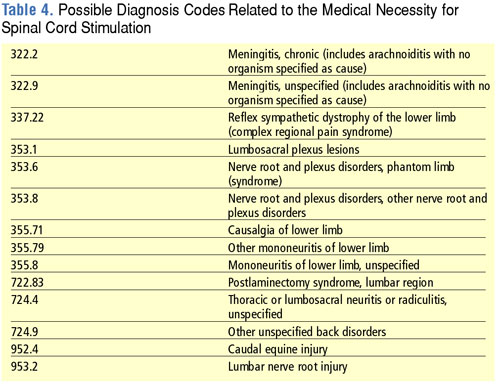
The 2006 HCPCS rates for neurostimulators can be downloaded at http://www.cms.hhs.gov/DMEPOS FeeSched/downloads/d06_jan_r.zip.
Based in information provided by Ms. Cascardo, President of Cascardo Consulting Group, on interviews with John Hernandez and Tom Walsh of Boston Scientific Corporation/Advanced Bionics, and on information available at www.con trolyourpain.com.

